How to Calculate Formal Charge
Directory of Chem Help ASAP videos. Using the formula to calculate the formal charge on hydrogen we obtain.
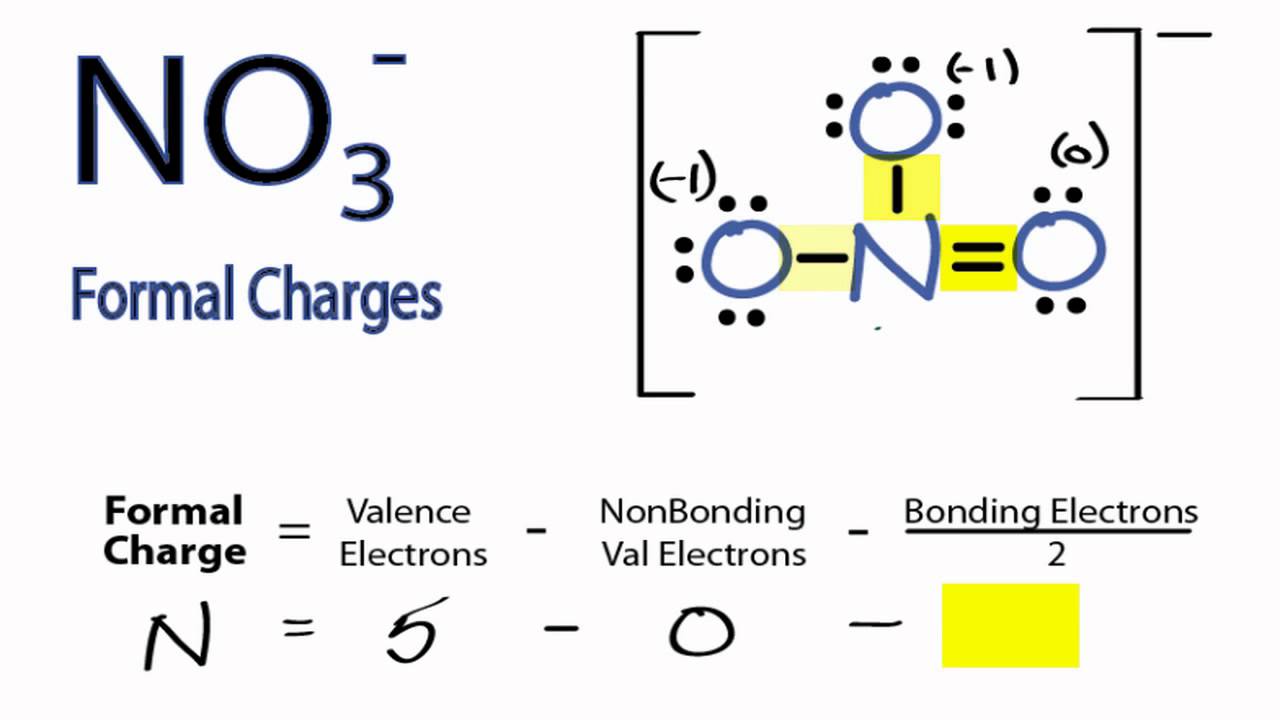
Calculating No3 Formal Charges Calculating Formal Charges For No3 Teaching Chemistry Chemistry Classroom Science Chemistry
FC Formal Charge on Atom.

. A step-by-step description on how to calculate formal charges. The formal charge on an atom in a molecule or ion is equal to the total number of valence electrons in the free atom minus the. You can calculate the formal charge of any atom with the help of the equation below.
The formal charges when added. Formal charge Valence Electrons Sticks Dots. Formula to Calculate the Formal Charge.
FC V LP 05BE Where. Formal charge exists because of deficiencies in the configuration of an atom that participates in the compound formation. Formal charge valence electrons nonbonding electrons 12 bonding electrons B Bonding electrons total electrons shared in.
Formal charge is the charge assigned to an atom in a molecule assuming that electrons in all chemical bonds are shared equally between atoms regardless of relative. FCO 6 ½ 6. Formal charges are important because they allow us to predict which Lewis structure is the mo.
Firstly we will find the formal charge on the sulphar S atom. Calculation of formal charge is the subject of this. The formula used to calculate formal charge.
To find the total formal charge of sulphate ion SO 42- we need to find the formal charge of all atoms individually. The formal charge of an atom is calculated by subtracting the number of electrons assigned to that atom in the Lewis structure from the number of protons in the nucleus of that atom. This chemistry video tutorial provides a basic introduction into how to calculate the formal charge of an atom or element in a lewis structure.
H3C S CH3 O Calculating some Formal Charges Sulfur is in Group IV. This way carbon has 4 oxygen has 6 and. Whereas if you have.
Using the formula charge formula for each atom present we can calculate the. The number of valence electrons equals to the elements group column in the periodic table. Charges Calculate the formal charge on the sulfur highlighted in red in the molecule shown.
CF group number of the atom - number of bonds it forms - number of unshared electrons If the atom has a CF with a value of 1 it is assigned a positive charge. V Number of. Calculate the formal charge of the compound using the Lewis Dot structure in step 1 and the formula given.
This organic chemistry video tutorial explains how to calculate the formal of an atom in a molecule using a simple formula. Formal charge H 1 valence e 0 nonbonding e 2 bonding e 2 0.

How To Calculate Formal Charge Education Help Science Lessons Organic Chemistry

How To Calculate Formal Charge In Organic Chemistry Video Chemistry Basics Organic Chemistry Chemistry

Practice Calculating Formal Charge With This Chemistry Sample Problem Persuasive Writing Prompts Chemistry Covalent Bonding Worksheet

Finding The Formal Charge Of An Atom Using This Very Easy Formula Youtube In 2022 Ap Chem Atom Formula

Organic Chemistry How To Calculate Formal Charge Video Organic Chemistry Chemistry Lessons Chemistry

How To Calculate Formal Charge Organic Molecules Organic Chemistry Organic Chem
Comments
Post a Comment